Quality and certifications
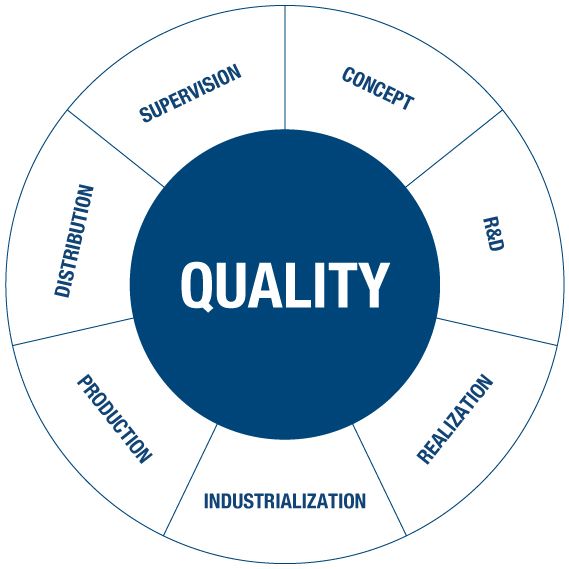
Quality in not an abstract concept. It is a real value that is at the centre of the Marco Viti Farmaceutici system and organisation.
a) It is a continuous process of production, control and quality improvement, applied at every stage of processing, in every sector and department.
b) It means satisfying the needs and expectations of the client and of the consumer.
c) It means operating in conformity with all health regulations regarding quality, safety and environment.
Our Certifications
- EN ISO 9001
Integrated quality management system. - EN ISO 22716
International quality standard for the production of cosmetics. - EN ISO 14001
Environmental certification. - EN ISO 45001
Certification for the health and safety of workers. - EN ISO 22000
International standard of food quality and safety for the production of dietary supplements. - EN ISO 13485
Integrated management system for quality in facilities for the production of medical devices. - cGMP - Good Manufacturing Practices
Authorisation AIFA (Italian Pharmaceutical Association) for the Group's pharmaceutical facilities for the production of medicines.